In this post our socio-legal researcher, Rachael Dickson, reflects on the interviews she has been conducting with persons living with attached and implanted medical devices.
The data collection phase of the Everyday Cyborgs 2.0 project commenced in earnest in November 2021. Nearly a year on, it seems like a good time to reflect on these encounters and the insights that have been gained through these activities. So far, we have conducted more than 40 interviews with persons living with attached and implanted medical devices. Interviewees encompass a diversity of ages, genders, geographical locations, and backgrounds. It has been a fascinating period of learning about what it is like to live with a medical device and the thoughts, opinions, preferences, worries, and recommendations that persons with devices hold.

The types of devices represented in our sample, thus far, fall into four broad categories: (1) devices that assist with the management of diabetes, (2) devices used to treat a range of cardiac conditions, (3) devices that address hearing loss, and (4) prosthetic limb devices. Initially, I wondered whether there would be any connections between the groups or whether the strength of individual identity of each would preclude any crossover. Whilst it is too early to say whether a genuine device users’ community exists, I was surprised by how frequently users of different devices raised similar issues.
DIYing Medical Devices
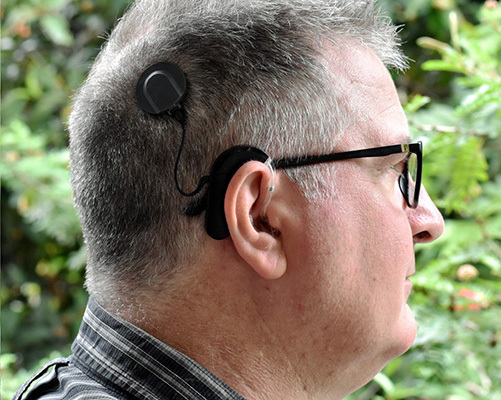
Most strikingly, for me, was that a significant number of interviewees with diabetes devices mentioned the phenomena of ‘Do-it-yourself’ artificial pancreas systems (also known as open source automated insulin delivery systems) (you can read more of our work on these here and here). Not every interviewee used one, but they all, to some degree, had considered the merits of this tech and explored the possibility of adopting it. Some were ardent supporters of the technology, describing how it had revolutionised their experience of managing their disease. Perhaps naively, I considered this as a unique situation and was surprised when a hearing aid user described buying an old programming machine from Ebay to try and develop his own settings. Prosthetic wearers have described DIY-ing their devices: embellishing their fixtures and fittings to not only provide greater comfort, but to improve the device’s functionality for certain activities – hiking, for example. Although cardiac device users didn’t directly describe altering the device, they did indicate an interest in having greater access to performance data, with some supplementing their knowledge through use of additional mass-market wearable devices.
A striking feature of the interviews was the prevalence of device users discussing making alternations or tweaks to their devices. I had expected to encounter these accounts amongst users of devices for the management of diabetes as the project had been examining the adoption of DIY APS/OS AID systems for a while. What was surprising was that this DIY trend appeared in relation to other devices. For example, a hearing aid user described buying an old programming machine from Ebay to try and develop custom settings. A person with a pacemaker described sourcing old pacemakers and home monitoring units to test the security protocols and see how hackable they were.
Prosthetic wearers described DIY-ing their devices too, not only provide greater comfort, but to improve the device’s functionality for certain activities – hiking, for example. Some of these augmentations could be described as essential, such as the user needing their prosthetic to fit correctly, but some could be seen as optional and about optimising the device. The question of the extent to which liability for harm caused as a result of DIY-ing a medical device has been prominent in relation to DIY APS, but with the DIY trend apparent in relation to other devices more analysis is needed about what the challenge of user-led augmentation of a device is and how the law ought to respond to these activities.
Empowerment, Advocacy, and Equitable Access to Devices
Broadly speaking these actions perhaps indicate a trend of EC’s taking ownership and responsibility for their personal health and wellbeing. Devices could be interpreted as empowering patients and, for some, providing an interface through which to exert preference and choice over how they manage their condition. This relates to another common thread emerging from the interviews, namely that with medical technology constantly evolving many of the device users we spoke with are engaged in patient advocacy. This can be via formal channels such as Cardiomyopathy UK’s ‘Change Maker’ role, grassroots movements like #WeAreNotWaiting, peer-to-peer support, or describing instances of self-advocating in interactions with healthcare professionals or the wider general public.
Underpinning this advocacy appears to be a commitment to enhancing the device user experience and a belief that medical device technology should further optimise what it can offer its users and push the boundaries of what is possible. Overall, most interviewees described their devices as having a positive impact on their lives, whether that was enabling them to live longer or live better.
However, that is not to say our interview participants do not have concerns. Devices becoming obsolete, the latest advancements being inaccessible due to cost, whether devices would be managed effectively in elderly care settings were raised as areas of worry. A common thing, which a number of participants brought up, was that they felt lucky to have their devices in a world where others did not have/are unable to get access to them. Increasing access to devices for those who want them and would benefit was expressed quite frequently.
Regulating Innovations
An area that perhaps our participants so far have not considered as much is the regulation of future innovations and where the dividing lines of responsibility should be drawn between the range of actors involved. While the clinical relationships that support their device use were often at the front of interviewees’ minds, there was some uncertainty expressed about the roles of regulators and manufacturers, with this increasing for less well-known roles like software developers, data-sharing platforms, marketing departments, and others. While the general view seems positive about the potential of future innovation, there appears to be a gap in opinion about how regulatory systems ought to respond and capture the changing nature of medical device technology.
Users appear to desire more transparency about the research underpinning their devices and greater access to information about how their information is used, as well as expressing interest in being more actively involved in the regulation of devices. With online device user communities offering a place for users to organise, develop their preferences, and empower individuals to advocate, I would argue that persons with medical devices will become a force that cannot be ignored. What will be interesting to explore in more depth is how user communities are changing the technological landscape, as well influencing law, regulation, and policy.
Capturing the Diversity of Users’ Experiences?
The challenge, however, is adequately capturing the range of preferences and experiences of the diverse populations and users with medical devices. Just as the range of devices is broad, there was a broad church of views expressed with regards how users experience their devices. For instance, some ardently expressed the view their device had been integrated with their person. It is part of them and is just who they are. Others gave their device its own personality, joking about giving it a name and conversing with it day-to-day. At the other end of the spectrum, some users maintained a more distanced respectfulness – acknowledging that the device is integral to their life, but viewing it more as a tool and not integrating it into their identity.
Overall, the empirical work has been an illuminating experience thus far. After a lack of interaction due to Covid, it has been fantastic to discuss our research with people again and hear a wide range of perspectives about life with a medical device. We hoped for a rich data set and we haven’t been disappointed by what we have achieved. The next step is to start determining what these experiences mean for the broader project questions and developing our analysis. That doesn’t mean the empirical work stops, though. We still hope to add perspectives from stakeholders and those working with medical devices in a professional capacity.
If this is you, please do get in touch with us at everyday.cyborgs@contacts.bham.ac.uk.
Written by: Rachael Dickson
Funding: Work on this was generously supported by a Wellcome Trust Investigator Award in Humanities and Social Sciences 2019-2024 (Grant No: 212507/Z/18/Z)
