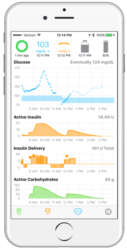
Some people with type 1 diabetes (T1D) find current treatment options to be inadequate and unsatisfactory and have taken it upon themselves to design technological solutions which work for them. Tired of waiting for commercial solutions that meet their needs, patients are constructing their own Do-it-Yourself Artificial Pancreas Systems (DIY APS). Although still confined to … Continue reading “Patients are doing it for themselves: DIY artificial pancreas systems and the challenge for doctors”
Month: March 2020
Briefing note: How we can improve the Medicines and Medical Devices Bill?
Currently, a large part of the UK’s regulatory regime governing medicines and medical devices is derived from EU law. The Medicines and Medical Devices Bill (MMDB) 2019-20 sets out a suite of provisions for the regulation of medicines and medical devices in the UK at the end of the EU exit transition period, currently 31 … Continue reading “Briefing note: How we can improve the Medicines and Medical Devices Bill?”