In this case study, we hear from Sandeep Das (based at the School of Chemistry), who has been utilising BEAR to design novel Li-ion battery materials.

I am a postdoctoral researcher and computational chemist in the Scanlon Materials Theory Group based at the School of Chemistry, University of Birmingham. My research is currently focussed on designing and understanding novel Li-ion battery cathode materials.
As the current Li-ion battery technologies in the market have mostly saturated in terms of electrochemical performance and capacity, newer and advanced cathode materials of varied composition and mechanisms are being investigated by battery researchers. This has become more important with the current push towards switching to renewable energy sources and technologies such as electric vehicles.
The RDS facility also facilitates the easy access and transfer of research data which makes the analysis part quite smooth
Disordered rock salt structures as cathodes are expected to feature high energy density and specific capacity compared to the currently used layered TM oxide cathodes. While the anionic site contains oxygen (O), the cation site as the name suggests is disordered due to a mixture of Li and one or more TMs. Li rich compositions are preferred to enhance Li transport through the formation of a Li percolating network. A redox inactive metal could be used to impart stability to the structure whereas another redox active TM along with O anions involve in the electrochemistry thereby contributing towards high capacity.
The various features of this computing facility such as availability of large number of cores per node and ability to run parallel calculations is very useful.
However, the O-redox process in these materials is a hotly debated topic. On one hand, while it is beneficial in terms of obtaining higher capacities, often, the oxidation of O anions can lead to release of O2 gas resulting in voltage hysteresis and irreversible phase transitions in the cathode material thereby decreasing the reversibility of the cathode material. Thus, it is essential that the O anion reduction is stabilised in a way so that the gas release can be prevented, which would then lead to a practical high-capacity cathode material. Hence, it is important to understand the electrochemical behaviour of O in a candidate DRX cathode material.
Overall, the compute facilities of BlueBEAR have allowed me to carry out my research at larger scales which are closer to practical scenarios and made them achievable at affordable times.
Computational chemistry using density functional theory (DFT) provides various methodologies for understanding battery mechanisms using the power of today’s state of the art high performance computer clusters. One way to understand O behaviour in cathode materials is to model representative structures at different points of discharge and observe the evolution of these structures using ab-initio molecular dynamics simulation (AIMD) which are supposed to be quite accurate. At different states of discharge, the cathode material will have vacancies due to removal of Li ions as the battery cycle progresses, thereby providing room for vacancies which can initiate the movement of various ions. This may lead to various processes like TM migration and O2 formation as the structure tries to stabilise.
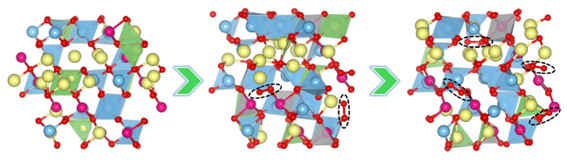
The AIMD simulations being very accurate are also quite resource heavy and time consuming, which limits the timescales up to which the systems could be studied, as well as number of atoms up to which models can be considered. Access to a powerful supercomputer like the in-house BlueBEAR in University of Birmingham is a privilege in carrying out these calculations. The various features of this computing facility, such as availability of large number of cores per node and ability to run parallel calculations is very useful. The availability of many cores per user also helps in timely calculation runs. The RDS facility also facilitates the easy access and transfer of research data which makes the analysis part quite smooth. The addition of Intel Sapphire nodes recently into the architecture has also been very useful to run faster calculations. Overall, the compute facilities of BlueBEAR have allowed me to carry out my research at larger scales which are closer to practical scenarios and made them achievable at affordable times.
To find out more about the work going on in the Scanlon Materials Theory Group head to Scanlon Materials Theory Group | Computationally Driven Materials Design (davidscanlon.com)
We were so pleased to hear of how Sandeep was able to make use of what is on offer from Advanced Research Computing, particularly to hear of how he has made use of BlueBEAR HPC and its storage – if you have any examples of how it has helped your research then do get in contact with us at bearinfo@contacts.bham.ac.uk.
We are always looking for good examples of use of High Performance Computing to nominate for HPC Wire Awards – see our recent winners for more details.
